Difference between revisions of "2022 DOCK tutorial 3 with PDBID 1X70"
BrockBoysan (talk | contribs) (→Energy Minimization for the ligand) |
BrockBoysan (talk | contribs) (→Surface Generation & Spheres) |
||
| Line 114: | Line 114: | ||
'''Sphere Selection''' | '''Sphere Selection''' | ||
| + | |||
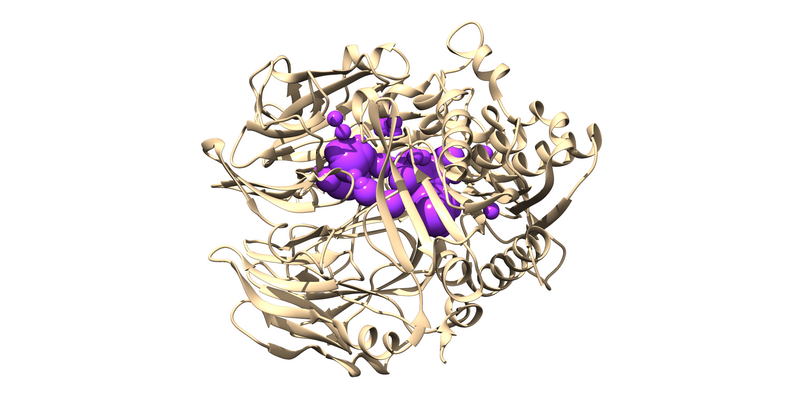
| + | [[File:1X70_sel_spheres.png|thumb|center|800px|The spheres we just selected to be using in docking experiments]] | ||
=Making The Infamous Grid= | =Making The Infamous Grid= | ||
Revision as of 20:50, 7 March 2022
Contents
Introduction
DOCK
System Preparation
Fetching 1X70
Open Chimera and do the following to grab the protein:
File > Fetch By ID > 1X70
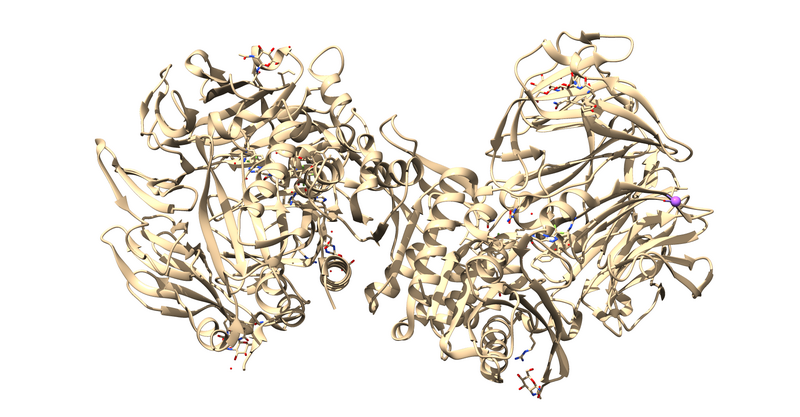
The first thing to notice is that this is a dimer and the ligand, 715, is not bound at the dimer interface. Thus, one of the monomers is entirely redundant and should be deleted.
Select > Chain > A
Actions > Atoms > Delete
Next it is important to remove cofactors, ions, and water molecules not involved in the binding interactions. This can be checked by reading through the paper associated with the PDB.
Select > Residue > NAG > Actions > Atoms > Delete
Select > Residue > NDG > Actions > Atoms > Delete
Select > Residue > HOH > Actions > Atoms > Delete
This will leave us with just the ligand and receptor.
Receptor Prep
Now that we the receptor by itself, we have to clean up the rest of the receptor by adding any missing side chains, dealing with multiple occupancies and mutated residues, and protonating and calculating partial charges.
For 1X70, there are several residues with multiple occupancies.
One way to check for these residues is to grep the number of alpha carbons from the pdb in the command line.
In the terminal, type: grep -e CA 1X70_noH.pdb
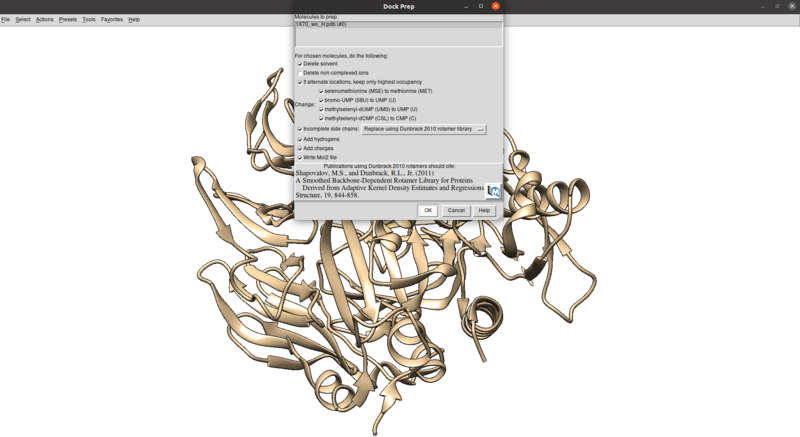
To clean this up and protonate/charge the receptor:
Tools > Structure Editing > Dock Prep
Make sure that the protonation makes sense for residues in the active site or coordinated with metals (none here), especially histidines, by checking the paper and chimera for any nitrogens coordinating with metals are not protonated or residues in the active site with differing protonation states. Also make sure that all residues have integral charge (i.e. the charge is an integer). Chimera should give a warning after the dockprep if any residue charges are non-integral.
Ligand Prep
First you have to save the ligand as a separate file. You can do this in Chimera by deleting all of the protein and saving that as a separate file: "715_noH.pdb".
Select > Residues > 715 > Invert Selection
Actions > Atoms > delete
File > save PDB
Now you should just have the ligand.
Next Hydrogens and partial atomic charges need to be added and saved as "715_H.mol2". It will ask for the ligands overall charge, which you should verify using chemical knowledge.
tools > structure editing > addH
tools > structure editing > addCharge > Select the correct charge for your ligand, Use AM1-BCC.
File > save Mol2
Surface Generation & Spheres
This section details the generation of sphere files which will be used to describe where you are trying to DOCK to on your protein.
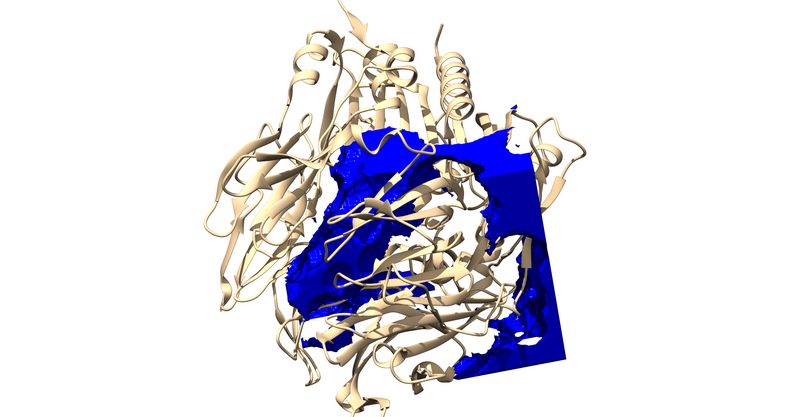
Surface Generation
Load 1X70 w/o Hydrogens in Chimera
actions > show > surface
Then you will write a DMS (Molecular Surface) File With the surface generated in Chimera:
Tools > Structure Editing > Write DMS
Save this as 1X70_dms.dms, and now you should have a DMS file for the next step.
Sphere Generation To generate spheres make the following input file: "INSPH" -
1X70_dms.dms #Molecular Surface File R #Whether to generate spheres outside of surface (R) or inside (L) X #Surface points from the DMS file to use in sphere generation 0 #Minimum radius between spheres 4.0 #Maximum radius of sphere 1.4 #Minimum radius of sphere 1X70_wo_H.sph #Output sphere file
For more information on sphere generation see: https://dock.compbio.ucsf.edu/DOCK_6/tutorials/sphere_generation/generating_spheres.htm
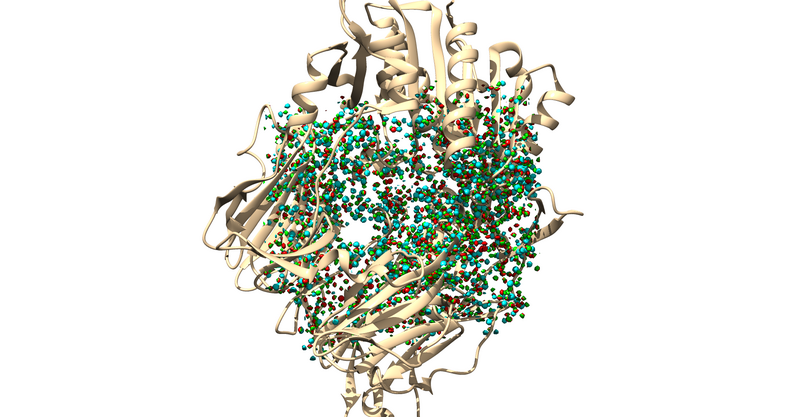
The .sph file should give you something similar to the following image if you load it up over your protein in Chimera:
Sphere Selection
Making The Infamous Grid
Making the box
In order to make the grid we first have to determine how big our grid will be. To do this:
cd 03.grid
showbox
#automatically construct box to enclose spheres [Y/N]
Y
#extra margin to also be enclosed (angstroms)?
#(this will be added in all 6 directions)
8.0
#sphere file-
./../02.spheres/selected_spheres.sph
#cluster number-
1
#output filename?
1X70.box.pdb
You can also copy these inputs into "showbox.in" (none of the commented lines) and then type "showbox < showbox.in"
Making the grid
Making the following input file "grid.in":
compute_grids yes grid_spacing 0.4 output_molecule no contact_score no energy_score yes energy_cutoff_distance 9999 atom_model a attractive_exponent 6 repulsive_exponent 9 distance_dielectric yes dielectric_factor 4. bump_filter yes bump_overlap 0.75 receptor_file ../01.structures/1X70_rec.mol2 box_file ./1X70.box.pdb vdw_definition_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/vdw_AMBER_parm99.defn score_grid_prefix grid
Now generate the grid. It should take several minutes.
grid -i grid.in -o gridinfo.out
Once it is done, vi into gridinfo.out and make sure the charges are all integer and that the calculation finished. If they are not, that likely means there is something wrong with your 1X70_H.mol2, and you may want to go back to the dockprep step and remake this file.
You should get two files:
grid.nrg (energy grid) grid.bmp (bump grid)
Try loading them into chimera over your protein.
Energy Minimization for the ligand
Now cd into directory 04.energy_min and make the following input file "min.in":
conformer_search_type rigid use_internal_energy yes internal_energy_rep_exp 12 internal_energy_cutoff 100.0 ligand_atom_file ./../01.structures/715.mol2 limit_max_ligands no skip_molecule no read_mol_solvation no calculate_rmsd yes use_rmsd_reference_mol yes rmsd_reference_filename ./../01.structures/715.mol2 use_database_filter no orient_ligand no bump_filter no score_molecules yes contact_score_primary no contact_score_secondary no grid_score_primary yes grid_score_secondary no grid_score_rep_rad_scale 1 grid_score_vdw_scale 1 grid_score_es_scale 1 grid_score_grid_prefix ./../03.grid/grid multigrid_score_secondary no dock3.5_score_secondary no continuous_score_secondary no footprint_similarity_score_secondary no pharmacophore_score_secondary no descriptor_score_secondary no gbsa_zou_score_secondary no gbsa_hawkins_score_secondary no SASA_score_secondary no amber_score_secondary no minimize_ligand yes simplex_max_iterations 1000 simplex_tors_premin_iterations 0 simplex_max_cycles 1 simplex_score_converge 0.1 simplex_cycle_converge 1.0 simplex_trans_step 1.0 simplex_rot_step 0.1 simplex_tors_step 10.0 simplex_random_seed 0 simplex_restraint_min yes simplex_coefficient_restraint 10.0 atom_model all vdw_defn_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/vdw_AMBER_parm99.defn flex_defn_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/flex.defn flex_drive_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/flex_drive.tbl ligand_outfile_prefix 1X70.lig.min write_orientations no num_scored_conformers 1 rank_ligands no
Then run this through dock
dock6 -i min.in -o min.out
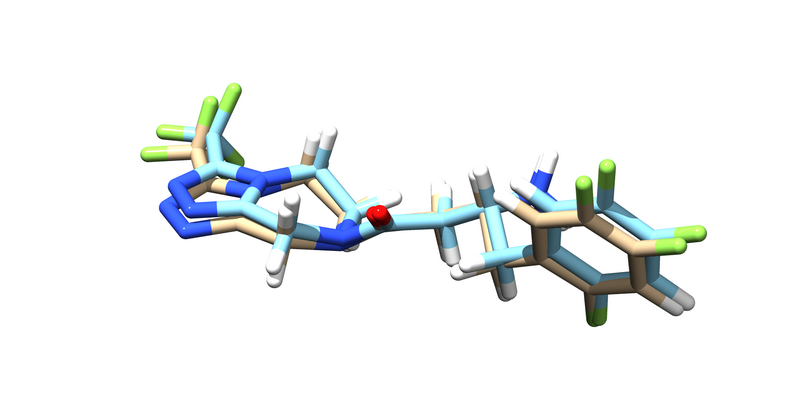
You should get an energy minimized version of the ligand. Try pulling it up over the original ligand in chimera.
Docking & Virtual Screening
Now that the ligand and receptor files are set up, we can begin docking the ligand back into the receptor. This makes sure that files are set up correctly, since we should expect the docking results to closely match the crystal structure from the PDB.
Rigid Docking
The first docking method we will be using is rigid docking. With this method, the ligand is kept in a fixed position while the algorithm tries to move/rotate the entire molecule to reduce energy. This is the least computationally intensive method for docking. We can start by creating the input file with the command
vim rigid.in
And using the following parameters.
conformer_search_type rigid use_internal_energy yes internal_energy_rep_exp 12 internal_energy_cutoff 100 ligand_atom_file 1x70.ligand.min_scored.mol2 limit_max_ligands no skip_molecule no read_mol_solvation no calculate_rmsd yes use_rmsd_reference_mol yes rmsd_reference_filename 1x70.ligand.min_scored.mol2 use_database_filter no orient_ligand yes automated_matching yes receptor_site_file ../002.surface_spheres/selected_spheres.sph max_orientations 2000 critical_points no chemical_matching no use_ligand_spheres no bump_filter no score_molecules yes contact_score_primary no contact_score_secondary no grid_score_primary yes grid_score_secondary no grid_score_rep_rad_scale 1 grid_score_vdw_scale 1 grid_score_es_scale 1 grid_score_grid_prefix ../003.gridbox/grid multigrid_score_secondary no dock3.5_score_secondary no continuous_score_secondary no footprint_similarity_score_secondary no pharmacophore_score_secondary no descriptor_score_secondary no gbsa_zou_score_secondary no gbsa_hawkins_score_secondary no SASA_score_secondary no amber_score_secondary no minimize_ligand yes simplex_max_iterations 1000 simplex_tors_premin_iterations 0 simplex_max_cycles 1 simplex_score_converge 0.1 simplex_cycle_converge 1.0 simplex_trans_step 1.0 simplex_rot_step 0.1 simplex_tors_step 10.0 simplex_random_seed 0 simplex_restraint_min no atom_model all vdw_defn_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/vdw_AMBER_parm99.defn flex_defn_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/flex.defn flex_drive_file /gpfs/projects/AMS536/zzz.programs/dock6.9_release/parameters/flex_drive.tbl ligand_outfile_prefix rigid.out write_orientations no num_scored_conformers 1 rank_ligands no
Fixed Anchor Docking
Flexible Docking
Placeholder
[[File:|thumb|center|800px|image placeholder]]