Difference between revisions of "Test Set Construction SB2024 V1 DOCK6.10 A"
(→III.Test Cases for Preparation Integrity) |
|||
| Line 73: | Line 73: | ||
|} | |} | ||
[[File:5UPG Zn HID crop.png|thumb|left]] | [[File:5UPG Zn HID crop.png|thumb|left]] | ||
| + | [[File:5UPG Zn HID crop.png|thumb|right]] | ||
| + | |||
==IV.To Do== | ==IV.To Do== | ||
Revision as of 21:06, 12 February 2024
!!!!!!Under Construction!!!!!!
The purpose of this tutorial is to develop a uniform method for adding systems to the Rizzo Lab test set, and rebuilding the set from initial files. This is the protocol to be used by all lab members. If the protocol is changed, a new tutorial should be made, preserving this tutorial.
Contents
- 1 I.Introduction
- 2 II.Scripts for System Preparation
- 3 A. Preliminary File Preparation
- 4 B. Ligand Preparation
- 5 C. Receptor Preparation
- 6 D. Sphere Generation
- 7 E. Single Grid Generation
- 8 F. Multi Grid Generation
- 9 G. Bookkeeping of Heavy Atoms
- 10 III.Test Cases for Preparation Integrity
- 11 IV.To Do
I.Introduction
A key development for this tutorial is bookkeeping scripts to manage the count of heavy atoms during processing and indicate unaccounted changes. Test cases are also included which should be visually inspected for indicated elements to be sure that processing occurred as expected.
II.Scripts for System Preparation
Each script should process the system in a fully automated manner. These scripts are located at (https://github.com/rizzolab/Testset_Protocols.git). There are key elements to check after each script before proceeding to the next. The output to be checked from each step is written to a folder "zzz.outfiles" with a unique file for each system, at each step. At the top of this file is the netid of the user who ran each process.
A. Preliminary File Preparation
The receptor and ligand should be prepared from the initial PDB file as outlined in student provided VS tutorials (https://ringo.ams.stonybrook.edu/index.php/Tutorials). Briefly:
(1) The ligand should have hydrogens added with careful attention to protonation state and gasteiger charges added. The extracted ligand is saved as "${pdb_id}.lig.moe.mol2" .
(2) The receptor is saved in isolation. Generally any water molecules are deleted, except specialized experiments. Any atomic ions within 8 angstroms of the ligand are retained as part of the receptor. The protocols have prep and frcmod files integrated for Heme cofactors, so these should be retained as part of the receptor. Once the receptor, metal ions, and heme cofactor are extracted they are saved together as "${pdb_id}.rec.foramber.pdb"
(3) Any biologically relevant organic small molecule cofactor (such as NADH / NADPH) other than Heme should be treated as a ligand (1) and saved as "${pdb_id}.cof.moe.mol2".
(4) Files from (1) (2) and (3) should be moved to the folder zzz.master in the upper folder Testset_Protocols from the github repo.
For further clarification, review the student tutorials as indicated.
B. Ligand Preparation
This step will assign am1bcc charges based on the charge in the preliminary file ${pdb_id}.lig.moe.mol2 and be saved as ${pdb_id}.lig.am1bcc.mol2
In the script submit.run.001.sh, if using a list of PDB codes other than "zzz.lists/clean.systems.all", change this in the script first.
sbatch submit.run.001.sh
C. Receptor Preparation
Due to an error in an Amber translation table P.3 atoms are converted to C.3 atoms when written to ${pdb_id}.rec.clean.mol2. At this point it is currently corrected manually, but is to be corrected in Amber23.
D. Sphere Generation
E. Single Grid Generation
F. Multi Grid Generation
G. Bookkeeping of Heavy Atoms
III.Test Cases for Preparation Integrity
These are cases which that need to be visually inspected to ensure key operations during preparation were executed. This step is crucial after building the test set in batch mode.
| System | Element | Details |
|---|---|---|
| 2GQG | PhosphoTyrosine Residue | Check Residue 171 correctly has Y2P phosphotyrosine (See Picture below) |
| 2Y03 | Disulfide Bond | Check bond Residue 82.SG and Residue 167.SG is actually bonded (See Picture below ) |
| 4WMZ | Heme | Check Heme is correctly incorporated, including Iron (Fe) |
| 1P44 | NADH Cofactor | Make sure cofactor is present in 1P44.rec.clean.mol2 and grid.out as residue "COF" |
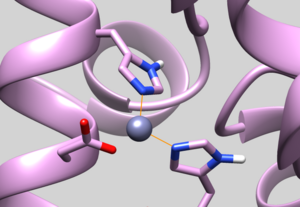
| 5UPG | Zinc Chelated Histidine Protonation | Residue should have HID protonation (See Picture below) |
| 3D94 | NonStandard Residue Defined Mutatation | Make sure Residue 146 successfully mutates to Residue "MET" from "MHO" |
| 1JWT | Long Bonds | Make sure TER is added before long bond |
IV.To Do
1. Integrate prep and frcmod files for cofactors available at http://amber.manchester.ac.uk/
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>
Tutorial Written By: Christopher Corbo, Rizzo Lab, Stony Brook University (2024)
>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>>