Difference between revisions of "2016 DOCK tutorial with Beta Trypsin"
Stonybrook (talk | contribs) (→IV. Generating Box and Grid) |
Stonybrook (talk | contribs) (→Box Generation) |
||
| Line 28: | Line 28: | ||
Monaf Awwa | Monaf Awwa | ||
=== Box Generation=== | === Box Generation=== | ||
| − | + | >Start Chimera and open the RECEPTOR files 1BJU.rec.noH.pdb | |
Next, select the ACTION subheader, then select SURFACE, then SHOW | Next, select the ACTION subheader, then select SURFACE, then SHOW | ||
| Line 35: | Line 35: | ||
Now that we have a surface, we can more effectively calculate regions of the protein where a small molecule can bind. To further accelerate calculations, we will specify which atoms to calculate the interactions for via the program sphgen | Now that we have a surface, we can more effectively calculate regions of the protein where a small molecule can bind. To further accelerate calculations, we will specify which atoms to calculate the interactions for via the program sphgen | ||
| + | |||
| + | Switch over to the 02.surfacespheres directory with the following command | ||
| + | |||
| + | cd 02.surfacespheres | ||
| + | |||
| + | Next, create an input file for sphgen to read. | ||
| + | |||
| + | vim INSPH | ||
| + | |||
| + | The file should have the following information | ||
| + | |||
| + | 1BJU.rec.dms #the file corresponding to the surface is the input file | ||
| + | R #we want the spheres outside the protein surface | ||
| + | X #we want all subsets of spheres to be generated | ||
| + | 0.0 #default size of proteins with surface contacts | ||
| + | 4.0 #default maximum of the sphere radius generated (Angstroms) | ||
| + | 1.4 #default minimum of the sphere radius generated (Angstroms) Also corresponds to probe radius | ||
| + | 1BJU.rec.sph #output file name | ||
| + | |||
| + | Now we can enter the command to run sphgen | ||
| + | |||
| + | sphgen -i INSPH -o OUTSPH | ||
| + | |||
| + | Once completed, check the OUTSPH file to ensure no errors occured | ||
| + | |||
| + | If you'd like to visually inspect the spheres generated, start Chimera and load the 1BJU.rec.mol2 file, then load the 1BJU.rec.sph file. | ||
| + | |||
| + | Now we will use the showsphere program to convert our .sph file back to a .pdb format. | ||
| + | |||
| + | In the same directory, enter the command | ||
| + | |||
| + | showsphere | ||
| + | |||
| + | The following questions will appear. Answer as follows | ||
| + | |||
| + | Enter name of sphere cluster file: | ||
| + | 1BJU.rec.sph | ||
| + | Enter cluster number to process (<0 = all): | ||
| + | -1 | ||
| + | Generate surfaces as well as pdb files (<N>/Y)? | ||
| + | N | ||
| + | Enter name for output file prefix: | ||
| + | output_spheres | ||
| + | Process cluster 0 (contains ALL spheres) (<N>/Y)? | ||
| + | N | ||
| + | |||
| + | You should now have a file named output_spheres.pdb in your directory. You can visualize the spheres by loading chimera and the 1BJU.rec.noH.mol2, then loading the output_spheres.pdb file. | ||
| + | |||
| + | For the docking calculation, we are most interesting in a subset of the spheres. To select the spheres most important to ligand binding, we will use the command | ||
| + | |||
| + | sphere_selector 1BJU.rec.sph ../01.dockprep/1BJU.lig.mol2 8.0 | ||
| + | |||
| + | The 8.0 Angstrom value indicates we want to select spheres within 8 angstroms of our ligand. This hypothetically means we are designing a competitive inhibitor. | ||
| + | |||
| + | Now lets create the proper output file for our spheres. Enter the command | ||
| + | |||
| + | showsphere | ||
| + | |||
| + | and answer the following questions | ||
| + | |||
| + | Enter name of sphere cluster file: | ||
| + | selected_spheres.sph | ||
| + | Enter cluster number to process (<0 = all): | ||
| + | -1 | ||
| + | Generate surfaces as well as pdb files (<N>/Y)? | ||
| + | N | ||
| + | Enter name for output file prefix: | ||
| + | output_spheres_selected | ||
| + | Process cluster 0 (contains ALL spheres) (<N>/Y)? | ||
| + | N | ||
| + | |||
| + | This will ensure the docking calculation samples the most important regions of the protein surface | ||
| + | |||
=== Grid computing=== | === Grid computing=== | ||
first, create an input file for sphgen to read. | first, create an input file for sphgen to read. | ||
Revision as of 14:35, 26 March 2016
For additional Rizzo Lab tutorials see DOCK Tutorials. Use this link Wiki Formatting as a reference for editing the wiki. This tutorial was developed collaboratively by the AMS 536 class of 2014, using DOCK v6.6.
Contents
I. Introduction
Yaping She & Omar Sanchez Reyes
DOCK
Beta Trypsin
Organizing Directories
II. Preparing the Receptor and Ligand
Beilei Jiang
III. Generating Receptor Surface and Spheres
Lauren Prentis
Generating the Receptor Surface
Placing Spheres
IV. Generating Box and Grid
Monaf Awwa
Box Generation
>Start Chimera and open the RECEPTOR files 1BJU.rec.noH.pdb Next, select the ACTION subheader, then select SURFACE, then SHOW
Once you can see the van der Waals surface of the protein, select the TOOLS subheader, then STRUCTURE EDITING, and lastly select WRITE DMS and save the file as 1BJU.dms
Now that we have a surface, we can more effectively calculate regions of the protein where a small molecule can bind. To further accelerate calculations, we will specify which atoms to calculate the interactions for via the program sphgen
Switch over to the 02.surfacespheres directory with the following command
cd 02.surfacespheres
Next, create an input file for sphgen to read.
vim INSPH
The file should have the following information
1BJU.rec.dms #the file corresponding to the surface is the input file R #we want the spheres outside the protein surface X #we want all subsets of spheres to be generated 0.0 #default size of proteins with surface contacts 4.0 #default maximum of the sphere radius generated (Angstroms) 1.4 #default minimum of the sphere radius generated (Angstroms) Also corresponds to probe radius 1BJU.rec.sph #output file name
Now we can enter the command to run sphgen
sphgen -i INSPH -o OUTSPH
Once completed, check the OUTSPH file to ensure no errors occured
If you'd like to visually inspect the spheres generated, start Chimera and load the 1BJU.rec.mol2 file, then load the 1BJU.rec.sph file.
Now we will use the showsphere program to convert our .sph file back to a .pdb format.
In the same directory, enter the command
showsphere
The following questions will appear. Answer as follows
Enter name of sphere cluster file:
1BJU.rec.sph
Enter cluster number to process (<0 = all):
-1
Generate surfaces as well as pdb files (<N>/Y)?
N
Enter name for output file prefix:
output_spheres
Process cluster 0 (contains ALL spheres) (<N>/Y)?
N
You should now have a file named output_spheres.pdb in your directory. You can visualize the spheres by loading chimera and the 1BJU.rec.noH.mol2, then loading the output_spheres.pdb file.
For the docking calculation, we are most interesting in a subset of the spheres. To select the spheres most important to ligand binding, we will use the command
sphere_selector 1BJU.rec.sph ../01.dockprep/1BJU.lig.mol2 8.0
The 8.0 Angstrom value indicates we want to select spheres within 8 angstroms of our ligand. This hypothetically means we are designing a competitive inhibitor.
Now lets create the proper output file for our spheres. Enter the command
showsphere
and answer the following questions
Enter name of sphere cluster file:
selected_spheres.sph
Enter cluster number to process (<0 = all):
-1
Generate surfaces as well as pdb files (<N>/Y)?
N
Enter name for output file prefix:
output_spheres_selected
Process cluster 0 (contains ALL spheres) (<N>/Y)?
N
This will ensure the docking calculation samples the most important regions of the protein surface
Grid computing
first, create an input file for sphgen to read.
vim INSPH
The file should have the following information. Remember that
V. Docking a Single Molecule for Pose Reproduction
Agatha Lyczek & Haoyue Guo
Minimization
Dock Minimization Input file: min.in
conformer_search_type rigid use_internal_energy yes internal_energy_rep_exp 12 ligand_atom_file ../01.dockprep/1BJU.lig.mol2 limit_max_ligands no skip_molecule no read_mol_solvation no calculate_rmsd yes use_rmsd_reference_mol yes rmsd_reference_filename ../01.dockprep/1BJU.lig.mol2 use_database_filter no orient_ligand no bump_filter no score_molecules yes contact_score_primary no contact_score_secondary no grid_score_primary yes grid_score_secondary no grid_score_rep_rad_scale 1 grid_score_vdw_scale 1 grid_score_es_scale 1 grid_score_grid_prefix ../03.box-grid/1BJU.grid multigrid_score_secondary no dock3.5_score_secondary no continuous_score_secondary no footprint_similarity_score_secondary no ph4_score_secondary no descriptor_score_secondary no gbsa_zou_score_secondary no gbsa_hawkins_score_secondary no SASA_descriptor_score_secondary no amber_score_secondary no minimize_ligand yes simplex_max_iterations 1000 simplex_tors_premin_iterations 0 simplex_max_cycles 1 simplex_score_converge 0.1 simplex_cycle_converge 1.0 simplex_trans_step 1.0 simplex_rot_step 0.1 simplex_tors_step 10.0 simplex_random_seed 0 simplex_restraint_min yes simplex_coefficient_restraint 10.0 atom_model all vdw_defn_file ../zzz.parameters/vdw.defn flex_defn_file ../zzz.parameters/flex.defn flex_drive_file ../zzz.parameters/flex_drive.tbl ligand_outfile_prefix 1BJU.min write_orientations no num_scored_conformers 1 rank_ligands no
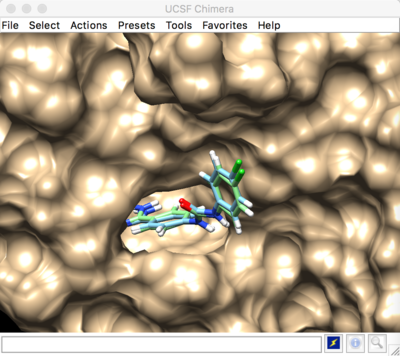
Result:
Rigid docking
To run the input file:
dock6 -i rigid_nr.in
Rigid docking input file: rigid_nr.in
conformer_search_type rigid use_internal_energy yes internal_energy_rep_exp 12 ligand_atom_file ../01.dockprep/1BJU.lig.mol2 limit_max_ligands no skip_molecule no read_mol_solvation no calculate_rmsd yes use_rmsd_reference_mol yes rmsd_reference_filename ../01.dockprep/1BJU.lig.mol2 use_database_filter no orient_ligand yes automated_matching yes receptor_site_file ../02.surface-sphere/selected_spheres.sph max_orientations 1000 critical_points no chemical_matching no use_ligand_spheres no bump_filter no score_molecules yes contact_score_primary no contact_score_secondary no grid_score_primary yes grid_score_secondary no grid_score_rep_rad_scale 1 grid_score_vdw_scale 1 grid_score_es_scale 1 grid_score_grid_prefix ../03.box-grid/1BJU.grid multigrid_score_secondary no dock3.5_score_secondary no continuous_score_secondary no footprint_similarity_score_secondary no ph4_score_secondary no descriptor_score_secondary no gbsa_zou_score_secondary no gbsa_hawkins_score_secondary no SASA_descriptor_score_secondary no amber_score_secondary no minimize_ligand yes simplex_max_iterations 1000 simplex_tors_premin_iterations 0 simplex_max_cycles 1 simplex_score_converge 0.1 simplex_cycle_converge 1.0 simplex_trans_step 1.0 simplex_rot_step 0.1 simplex_tors_step 10.0 simplex_random_seed 0 simplex_restraint_min yes atom_model all vdw_defn_file ../zzz.parameters/vdw.defn flex_defn_file ../zzz.parameters/flex.defn flex_drive_file ../zzz.parameters/flex_drive.tbl ligand_outfile_prefix 1BJU.rigid_nr write_orientations no
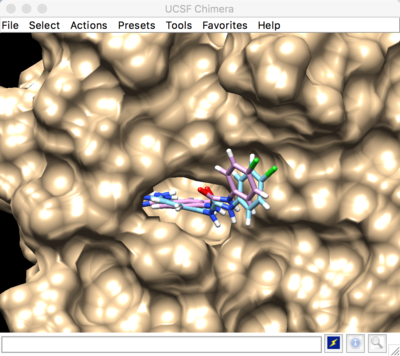
Best scored rigid docking result:
Flexible Docking
To run the input file:
dock6 -i flex.in
Flexible docking input file: flex.in
conformer_search_type flex user_specified_anchor no limit_max_anchors no min_anchor_size 5 pruning_use_clustering yes pruning_max_orients 1000 pruning_clustering_cutoff 100 pruning_conformer_score_cutoff 100.0 use_clash_overlap no write_growth_tree no use_internal_energy yes internal_energy_rep_exp 12 ligand_atom_file ../01.dockprep/1BJU.lig.mol2 limit_max_ligands no skip_molecule no read_mol_solvation no calculate_rmsd yes use_rmsd_reference_mol yes rmsd_reference_filename ../01.dockprep/1BJU.lig.mol2 use_database_filter no orient_ligand yes automated_matching yes receptor_site_file ../02.surface-sphere/selected_spheres.sph max_orientations 1000 critical_points no chemical_matching no use_ligand_spheres no bump_filter no score_molecules yes contact_score_primary no contact_score_secondary no grid_score_primary yes grid_score_secondary no grid_score_rep_rad_scale 1 grid_score_vdw_scale 1 grid_score_es_scale 1 grid_score_grid_prefix ../03.box-grid/1BJU.grid multigrid_score_secondary no dock3.5_score_secondary no continuous_score_secondary no footprint_similarity_score_secondary no ph4_score_secondary no descriptor_score_secondary no gbsa_zou_score_secondary no gbsa_hawkins_score_secondary no SASA_descriptor_score_secondary no amber_score_secondary no minimize_ligand yes minimize_anchor yes minimize_flexible_growth yes use_advanced_simplex_parameters no simplex_max_cycles 1 simplex_score_converge 0.1 simplex_cycle_converge 1.0 simplex_trans_step 1.0
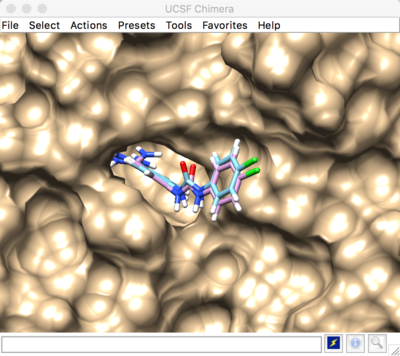
Best scored flexible docking result:
VI. Virtual Screening
Katie Maffucci
DOCK Preparation
Post Processing
VIII. Frequently Encountered Problems
EVERYONE