2024 AMBER tutorial 2 with PDBID 1NDV
Contents
Introduction
DOCK6 is a great tool that helps us understand ligand binding poses and relative binding energies. When DOCK6 is combined with another computational method known as molecular dynamics, we can calculate free energies of binding and probe how the ligand behaves in the binding pocket. In this tutorial we will walk through the use of AMBER16 to perform and analyze molecular dynamic simulations of PDBID 1NDV.
Getting Started
Before starting simulations it is important to create our directories so that we can keep our simulations organized. Create the following directories:
000.paramters 001.tleap_build 002.equilbration 003.production 004.analysis zzz.master
We will use the zzz.master directory to store our initial mol2 files and other files we may use that do not belong in another directory. Once you have your directories created you can begin to prepare they system for simulation.
Before Simulation
Structure
We will need mol2 and pdb files of the ligand and receptor separately for these simulations. If you followed the 1NDV DOCK6 VS tutorial you will already have these files and can move them to the zzz.master directory. If you did not follow that tutorial the steps will be listed below, but please refer to it if you require a more in depth tutorial. Please note that the receptor file should be without charges and hydrogens, while the ligand will need the charges and hydrogens.
We will start by generating our receptor file. Open the PDB of 1NDV in Chimera and follow these steps.
1) Select an atom on the receptor and use the entire up arrow until the entire protein is selected 2) Select -> Invert (all models) 3) Actions -> Atoms/Bonds -> delete
This should leave us with just the receptor atoms. Save this as a mol2 file and a pdb file as "1ndv_protein_noH_noCharge" DO NOT add charges or hydrogens.
We will now create the ligand file in a similar fashion.
1) Select an atom on the ligand and use the entire up arrow until the entire protein is selected 2) Select -> Invert (all models) 3) Actions -> Atoms/Bonds -> delete
Before we use this file we must add hydrogens and charges.
To add Hydrogens: Tools -> Structure Editing -> Add Hydrogens To add Charges: Tools -> Structure Editing -> Add Charges (for this ligand we will use an overall charge of 0)
Be sure to reference the PDB file to ensure that Chimera adds hydrogens in the correct places. Once charges and hydrogens are added you can save the mol2 and pdb files as "1ndv_ligand_addH_addCharge" Place both of these files in the zzz.master directory.
Force Field Parameters
Before we can use TLEap, we need to generate forcefield parameters for our ligand. We will use the antechamber program to do this. Move to the 000.paramters directory and use the following command to generate the forcefield parameter file.
antechamber -i ../zzz.master/1ndv_ligand_addH_addCharge.mol2 -fi mol2 -o 1ndv_ligand_antechamber.mol2 -fo mol2 -at gaff -c bcc -rn LIG
When running this you may encounter some warnings such as:
Warning: The number of bonds (3) for atom (ID: 1, Name: N1) does not match the connectivity (2) for atom type (N.ar) defined in CORR_NAME_TYPE.DAT.
Be sure to double check your ligand has the correct bond connectivity, and then these warnings may be safely ignored. You may also encounter some errors that prevent antechamber from running. It may require you to enter the mol2 file and edit the atom types to resolve them. Once antechamber has successfully run, run the following command to make our modified forcefield parameters:
parmchk2 -i 1ndv_ligand_antechamber.mol2 -f mol2 -o 1ndv_ligand.am1bcc.frcmod
TLEap
Now that we have our modified forcefield parameters we can use TLEap to solvate and prepare the system for simulation. Create a file called "tleap.build.in" and add the folowing commands:
#!/usr/bin/sh
###load protein force field
source leaprc.protein.ff14SB
###load GAFF force field (for our ligand)
source leaprc.gaff
###load TIP3P (water) force field
source leaprc.water.tip3p
###load ions frcmod for the tip3p model
loadamberparams frcmod.ionsjc_tip3p
###needed so we can use igb=8 model
set default PBradii mbondi3
###load protein pdb file
rec=loadpdb ../001.structure/1ndv_built.pdb
#THIS IS WHERE YOU WOULD DEFINE DISULFIDE BONDS
#NUMBERING SHOULD MATCH INPUT PDB FILE
#bond rec.Res#.SG rec.Res#.SG
###load ligand frcmod/mol2
loadamberparams ../002.parameters/1ndv_ligand.am1bcc.frcmod
lig=loadmol2 ../002.parameters/1ndv_ligand_antechamber.mol2
###create gase-phase complex
gascomplex= combine {rec lig}
###write gas-phase pdb
savepdb gascomplex 1ndv.gas.complex.pdb
###write gase-phase toplogy and coord files for MMGBSA calc
saveamberparm gascomplex 1ndv.complex.parm7 1ndv.gas.complex.rst7
saveamberparm rec 1ndv.gas.receptor.parm7 1ndv.gas.receptor.rst7
saveamberparm lig 1ndv.gas.ligand.parm7 1ndv.gas.ligand.rst7
###create solvated complex (albeit redundant)
solvcomplex= combine {rec lig}
###solvate the system
solvateoct solvcomplex TIP3PBOX 12.0
###Neutralize system
addions solvcomplex Cl- 0
addions solvcomplex Na+ 0
#write solvated pdb file
savepdb solvcomplex 1ndv.wet.complex.pdb
###check the system
charge solvcomplex
check solvcomplex
###write solvated toplogy and coordinate file
saveamberparm solvcomplex 1ndv.wet.complex.prmtop 1ndv.wet.complex.rst7
quit
Be sure that all files are generated, especially the wet.complex files, and review the tleap_output.txt file to ensure that no errors were encountered. We will also load the wet.complx files into Chimera to make sure TLEaP ran properly. Download the 1ndv.wet.complex.prmtop and 1ndv.wet.complex.rst7. Now in Chiemra:
Tools → MD/Ensemble Analysis → MD Movie
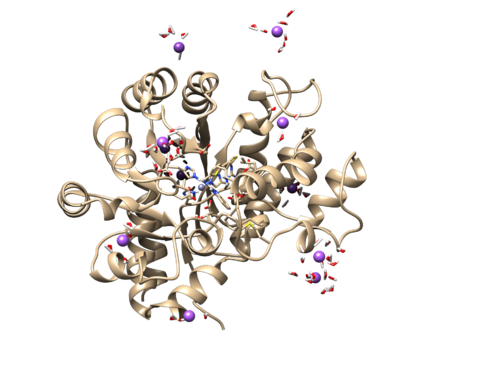
We will use the rst7 file as our trajectory for this step. It should load an image similar to this.
Now lets make sure that the rest of the waters were added properly and that our complex is in the solvent box. To do this:
1) Select → Chain → water 2) Actions → Atoms/Bond → Show
This should show all the waters added by TLEaP and should look similar to this.
Minimization and Equilibration
Before we run a production simulation, we must first minimize and equilibrate the system. We will do this with a 9-step procedure. This process will minimize the energy of the structure, slowly heat the system to the desired temperature, and then equilibrate the system at that temperature. First navigate the the 002.equiilibration directory. As we create the input files pay attention to the "restrainmask" variable. We want to restrain the protein and ligand atoms but not the hydrogens added by TLEaP so this will be set to ":1-350 & !@H=" for our system but be sure to change this is you are using a different receptor and ligand. This will change to just the protein atoms i.e. ":1-349 & !@H=" after step 7.
Create a file 01.min.mdin and add the following lines:
Minmize all the hydrogens &cntrl imin=1, ! Minimize the initial structure maxcyc=5000, ! Maximum number of cycles for minimization ntb=1, ! Constant volume ntp=0, ! No pressure scaling ntf=1, ! Complete force evaluation ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=5.0, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format /
Create a file 02.equil.mdin and add the following lines:
MD simualation &cntrl imin=0, ! Perform MD nstlim=50000 ! Number of MD steps ntb=2, ! Constant Pressure ntc=1, ! No SHAKE on bonds between hydrogens dt=0.001, ! Timestep (ps) ntp=1, ! Isotropic pressure scaling barostat=1 ! Berendsen taup=0.5 ! Pressure relaxtion time (ps) ntf=1, ! Complete force evaluation ntt=3, ! Langevin thermostat gamma_ln=2.0 ! Collision Frequency for thermostat ig=-1, ! Random seed for thermostat temp0=298.15 ! Simulation temperature (K) ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-235 & !@H=", ! atoms to be restrained restraint_wt=5.0, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format iwrap=1, ! iwrap is turned on /
Create a file 03.min.mdin and add the following lines:
Minmize all the hydrogens &cntrl imin=1, ! Minimize the initial structure maxcyc=1000, ! Maximum number of cycles for minimization ntb=1, ! Constant volume ntp=0, ! No pressure scaling ntf=1, ! Complete force evaluation ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=2.0, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format /
Create a file 04.min.mdin and add the following lines:
Minmize all the hydrogens &cntrl imin=1, ! Minimize the initial structure maxcyc=1000, ! Maximum number of cycles for minimization ntb=1, ! Constant volume ntp=0, ! No pressure scaling ntf=1, ! Complete force evaluation ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=0.1, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format /
Create a file 05.min.mdin and add the following lines:
Minmize all the hydrogens &cntrl imin=1, ! Minimize the initial structure maxcyc=1000, ! Maximum number of cycles for minimization ntb=1, ! Constant volume ntp=0, ! No pressure scaling ntf=1, ! Complete force evaluation ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=0.05, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format /
Create a file 06.equil.mdin and add the following lines:
MD simualation &cntrl imin=0, ! Perform MD nstlim=50000 ! Number of MD steps ntb=2, ! Constant Pressure ntc=1, ! No SHAKE on bonds between hydrogens dt=0.001, ! Timestep (ps) ntp=1, ! Isotropic pressure scaling barostat=1 ! Berendsen taup=0.5 ! Pressure relaxtion time (ps) ntf=1, ! Complete force evaluation ntt=3, ! Langevin thermostat gamma_ln=2.0 ! Collision Frequency for thermostat ig=-1, ! Random seed for thermostat temp0=298.15 ! Simulation temperature (K) ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=1.0, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format iwrap=1, ! iwrap is turned on /
Create a file 07.equil.mdin and add the following lines:
MD simulation &cntrl imin=0, ! Perform MD nstlim=50000 ! Number of MD steps ntx=5, ! Positions and velocities read formatted irest=1, ! Restart calculation ntc=1, ! No SHAKE on for bonds with hydrogen dt=0.001, ! Timestep (ps) ntb=2, ! Constant Pressure ntp=1, ! Isotropic pressure scaling barostat=1 ! Berendsen taup=0.5 ! Pressure relaxtion time (ps) ntf=1, ! Complete force evaluation ntt=3, ! Langevin thermostat gamma_ln=2.0 ! Collision Frequency for thermostat ig=-1, ! Random seed for thermostat temp0=298.15 ! Simulation temperature (K) ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-350 & !@H=", ! atoms to be restrained restraint_wt=0.5, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format iwrap=1, ! iwrap is turned on /
Create a file 08.equil.mdin and add the following lines:
MD simulations &cntrl imin=0, ! Perform MD nstlim=50000 ! Number of MD steps ntx=5, ! Positions and velocities read formatted irest=1, ! Restart calculation ntc=1, ! No SHAKE on for bonds with hydrogen dt=0.001, ! Timestep (ps) ntb=2, ! Constant Pressure ntp=1, ! Isotropic pressure scaling barostat=1 ! Berendsen taup=0.5 ! Pressure relaxtion time (ps) ntf=1, ! Complete force evaluation ntt=3, ! Langevin thermostat gamma_ln=2.0 ! Collision Frequency for thermostat ig=-1, ! Random seed for thermostat temp0=298.15 ! Simulation temperature (K) ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-349@CA.C.N", ! atoms to be restrained, only the backbone restraint_wt=0.1, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format iwrap=1, ! iwrap is turned on /
Create a file 09.equil.mdin and add the following lines:
MD simulations &cntrl imin=0, ! Perform MD nstlim=50000 ! Number of MD steps ntx=5, ! Positions and velocities read formatted irest=1, ! Restart calculation ntc=1, ! No SHAKE on for bonds with hydrogen dt=0.001, ! Timestep (ps) ntb=2, ! Constant Pressure ntp=1, ! Isotropic pressure scaling barostat=1 ! Berendsen taup=0.5 ! Pressure relaxtion time (ps) ntf=1, ! Complete force evaluation ntt=3, ! Langevin thermostat gamma_ln=2.0 ! Collision Frequency for thermostat ig=-1, ! Random seed for thermostat temp0=298.15 ! Simulation temperature (K) ntwx= 1000, ! Write to trajectory file every ntwx steps ntpr= 1000, ! Print to mdout every ntpr steps ntwr= 1000, ! Write a restart file every ntwr steps cut= 8.0, ! Nonbonded cutoff in Angstroms ntr=1, ! Turn on restraints restraintmask=":1-349@CA.C.N", ! atoms to be restrained restraint_wt=0.1, ! force constant for restraint ntxo=1, ! Write coordinate file in ASCII format ioutfm=0, ! Write trajectory file in ASCII format iwrap=1, ! iwrap is turned on /
This should not be run on the head node, instead create a submission script called equil.slurm and add the following lines:
#! /bin/sh #SBATCH --job-name=sys_equilibration #SBATCH --ntasks-per-node=40 #SBATCH --nodes=2 #SBATCH --time=8:00:00 #SBATCH -p long-40core
cd $SLURM_SUBMIT_DIR
echo "Started Equilibration on `date` " do_parallel="mpirun pmemd.MPI"
prmtop="../001.tleap_build/2nnq.wet.complex.prmtop" coords="../001.tleap_build/2nnq.wet.complex"
MDINPUTS=(01.min 02.equil 03.min 04.min 05.min 06.equil 07.equil 08.equil 09.equil)
for input in ${MDINPUTS[@]}; do
$do_parallel -O -i ${input}.mdin -o ${input}.mdout -p $prmtop -c ${coords}.rst7 -ref ${coords}.rst7 -x ${input}.trj -inf ${input}.info -r ${input}.rst7
coords=$input
done
echo "Finished Equilibration on `date` "